【骨鬆Q&A 006】誰應該接受藥物治療骨質疏鬆症?
根據葡萄牙風濕病學會(the Portuguese Society of Rheumatology,SPR)在2018年的建議 (Rodrigues AM 2018 )。
|
建議5 (Rodrigues AM 2018) 除非有禁忌症,否則在50歲以上滿足以下一項或多項要求的所有受試者中應開始對骨質疏鬆症進行藥理治療
標準:
|
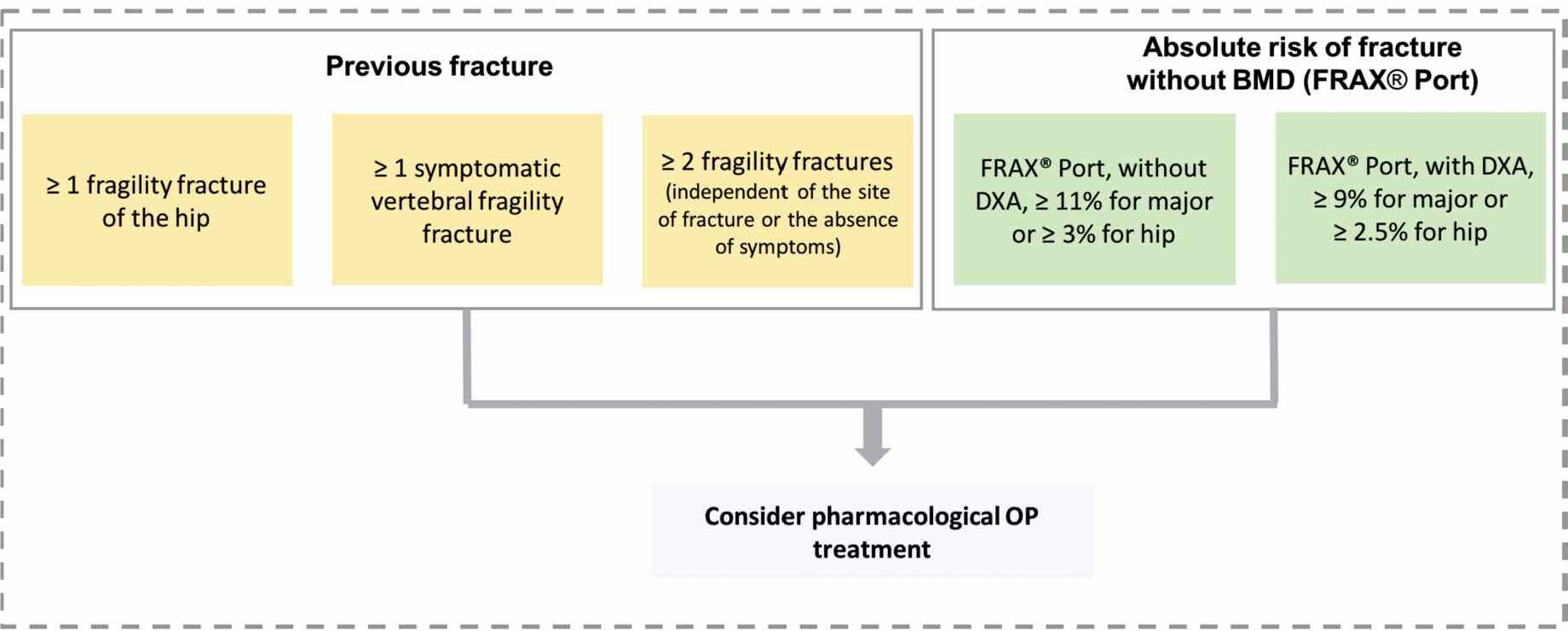
【骨鬆Q&A 005】我們知道使用BMD 作為單一評估標準是有問題的。這個問題出現在診斷過程及考慮需不需要開始藥物治療。現在我們來看看學理上何時應該開始藥物治療。
SPR認為開不開抗骨質疏鬆藥物的決定,應該基於FRAX®Port工具估計的個體隨後發生骨質疏鬆性骨折的十年風險。
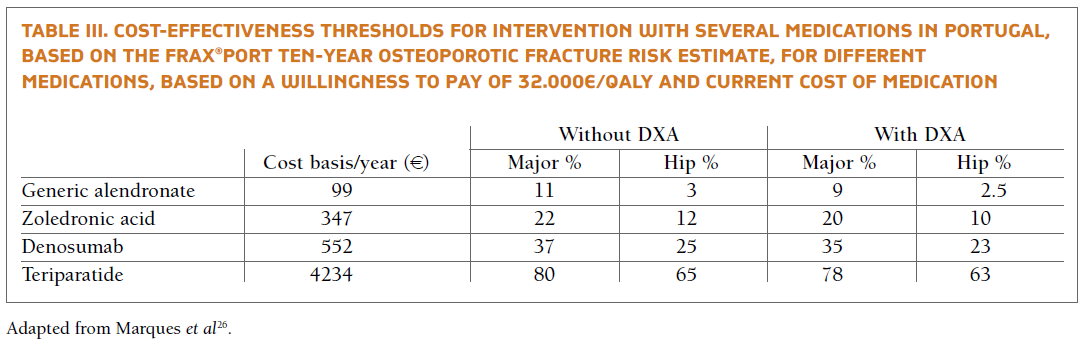
上述治療風險閾值基於成本效益分析,適用於最經濟的治療方案:通用Alendronate。
更昂貴的藥物具有更高成本效益的干預門檻 (Marques A 2016)。
先前脆性骨折的患者(尤其是髖關節)將顯著降低藥物治療後發生脆性骨折的風險,而與骨密度無關 (VERT FIT trial) (Black DM 1996, Harris ST 1999, Reginster J 2000, Neer RM 2001, Chesnut CH 2004, Kanis JA 2005, Lyles KW 2007, Cummings SR 2009)。小魏醫師再次強調骨質疏鬆症的治療是在降低骨折風險,而不是治療骨質密度,這是基層醫師最常犯的錯誤。
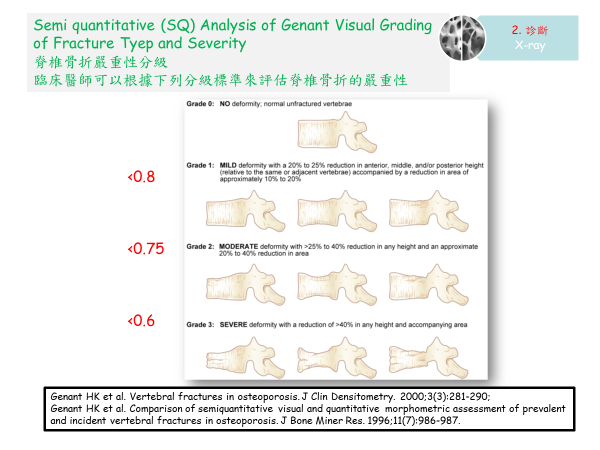
值得注意的是,一些國際建議,即使無症狀,在出現椎體畸形2級(即錐體高度減少> 25-40%)的情況下也應開始治療 (Black DM 2000)。
專家們意識到許多國際建議表明DXA T評分≤-2.5的患者也應接受治療,不論FRAX®和年齡 (Papaioannou A 2010, Kanis JA 2013, Cosman F 2014, Camacho PM 2016, Rossini M 2016)。
這些建議是基於這樣一個原則,即是在藥物治療的基礎下,減少股骨頸或腰椎T值達到-2.5或更低的骨折風險 (Reginster J 2000, McClung MR 2001, Neer RM 2001, Rossouw JE 2002, Sorensen OH 2003, Anderson GL 2004, Bone HG 2004, Miller PD 2005, Black DM 2006, Reginster JY 2006, Black DM 2007, Lyles KW 2007, Eisman JA 2008, Cummings SR 2009)。
根據葡萄牙多學科建議,SPR不認可這一政策,因為低BMD不一定與骨折的重大風險相關,特別是在年輕人中 (Marshall D 1996, Johansson H 2009)。
小魏醫師總結,國際專家對於治療與否,專注在幾個因子,包括FRAX、BMD、fragility fracture脆性骨折。其中以FRAX最為重要,因為它包含一般的危險因子評估,一次作齊,很少疾病有如此方便的大數據評估工具。https://www.sheffield.ac.uk/FRAX/tool.aspx?country=26
一旦FRAX接近治療閥值,再進一步評估BMD及骨折狀況,根據國家健康政策規範治療。
【參考資料】
- Anderson GL, L. M., Assaf AR, (2004). "Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial." JAMA 291(14): 1701- -1712.
- Black DM, C. S., Karpf DB, (1996). "Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group." Lancet 348(9041): 1535--1541.
- Black DM, D. P., Eastell R, (2007). "Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis." N Engl J Med 356(18): 1809-1822.
- Black DM, S. A., Ensrud KE, (2006). "Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial." JAMA 296(24): 2927-2938.
- Black DM, T. D., Bauer DC, (2000). "Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group." J Clin Endocrinol Metab 85(11): 4118-4124.
- Bone HG, H. D., Devogelaer JP, (2004). "Ten years’ experience with alendronate for osteoporosis in postmenopausal women." N Engl J Med 350(12): 1189- -1199.
- Camacho PM, P. S., Binkley N, (2016). "American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteo porosis - 2016." Endocr Pract. 22(Suppl 4): 1--42.
- Chesnut CH, r., Skag A, Christiansen C, (2004). "Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis." J Bone Miner Res 19(8): 1241-1249.
- Cosman F, d. B. S., LeBoff MS, (2014). "Clinician’s Guide to Prevention and Treatment of Osteoporosis." Osteoporos Int 25(10): 2359-2381.
- Cummings SR, S. M. J., McClung MR, (2009). "Denosumab for prevention of fractures in postmenopausal women with osteoporosis." N Engl J Med 361(8): 756-765.
- Eisman JA, C. R., Adami S, (2008). "Efficacy and tole rability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study." J Rheumatol. 35(3): 488-497.
- Harris ST, W. N., Genant HK, (1999). "Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group." JAMA 282(14): 1344-1352.
- Johansson H, K. J., Oden A, Johnell O, McCloskey E, (2009). "BMD, clinical risk factors and their combination for hip fracture prevention." Osteoporos Int 20(10): 1675-1682.
- Kanis JA, B. I., Johnell O, (2005). "Risedronate decreasesfracture risk in patients selected solely on the basis of prior vertebral fracture." Osteoporos Int 16(5): 475-482.
- Kanis JA, M. E., Johansson H, (2013). "European guidance for the diagnosis and management of osteoporosis in postmenopausal women." Osteoporos Int 24(1): 23-57.
- Lyles KW, C.-E. C., Magaziner JS, (2007). "Zoledronic acid and clinical fractures and mortality after hip fracture." N Engl J Med 357(18): 1799-1809.
- Marques A, L. O., Ortsater G, Borgstrom F, Kanis JA, da Silva JA, (2016). "Cost-Effectiveness of Intervention Thresholds for the Treatment of Osteoporosis Based on FRAX((R)) in Portugal." Calcif Tissue Int 99(2): 131-141.
- Marshall D, J. O., Wedel H, (1996). "Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures." BMJ 312(7041): 1254-1259.
- McClung MR, G. P., Miller PD, (2001). "Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group." N Engl J Med 344(5): 333-340.
- Miller PD, M. M., Macovei L, (2005). "Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study." J Bone Miner Res 20(8): 1315-1322.
- Neer RM, A. C., Zanchetta JR, (2001). "Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis." N Engl J Med 344(19): 1434-1441.
- Papaioannou A, M. S., Cheung AM, (2010). "2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary." CMAJ 182(17): 1864-1873.
- Reginster J, M. H., Sorensen OH, (2000). "Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group." Osteoporos Int 11(1): 83--91.
- Reginster JY, A. S., Lakatos P, (2006). "Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study." Ann Rheum Dis 65(5): 654-661.
- Rodrigues AM (2018). "Portuguese recommendations for the prevention, diagnosis and management of primary osteoporosis – 2018 update." ACTA REUMATOL PORT 43: 10-31.
- Rodrigues AM, Canhão H, Marques A, Ambrósio C, Borges J, (2018 ). "Portuguese recommendations for the prevention, diagnosis and management of primary osteoporosis - 2018 update." Acta Reumatol Port 43(1): 10-31.
- Rossini M, A. S., Bertoldo F, (2016). "Guidelines for the diagnosis, prevention and management of osteoporosis." Reumatismo 68(1): 1-39.
- Rossouw JE, A. G., Prentice RL, (2002). "Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial." JAMA 288(3): 321-333.
- Sorensen OH, C. G., Mulder H, (2003). "Long-term efficacy of risedronate: a 5-year placebo-controlled clinical experience." Bone 32(2): 120-126.